Whether a Reaction Is Exothermic or Endothermic Is Determined by
If the equation has energy or heat on the left hand side of the reaction arrow the reaction is endothermic. Combustion neutralisation displacement condensation.

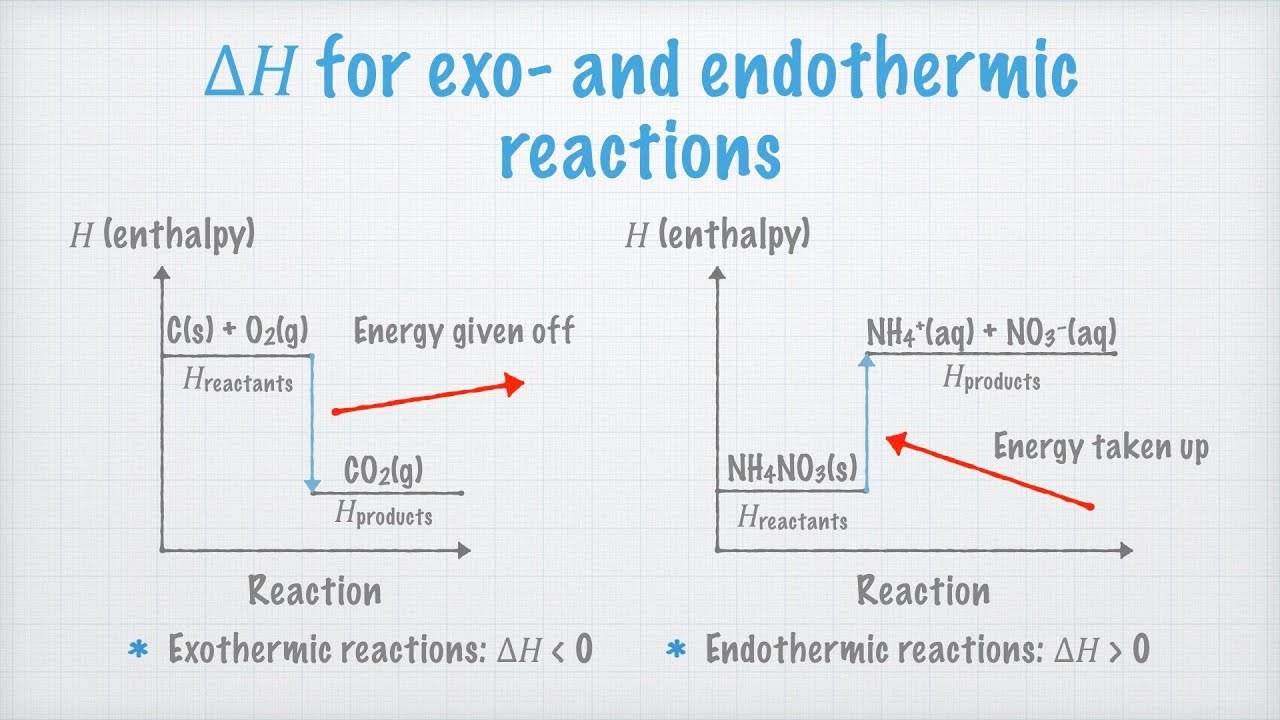
Heat Of Reaction Reflects The Difference In Enthalpy Between The Products And The Reactants Teaching Chemistry Chemistry Education Teaching Science
For the reaction N 2 O 4 2 NO 2 a bond between the two nitrogens is broken apart.

. Here are some ways to determine if a reaction is endothermic. A the activation energy B the physical state of the reactants C how reactant and product potential energies compare D whether a catalyst is present E the magnitude of the Gibbs free energy. Endothermic reactions have reactants with higher energy and products with lower energy.
Determining if a Reaction is Endothermic or Exothermic There is one step we must go through to determine if a reaction is endothermic or exothermic. A reaction that converts chemical energy to thermal energy heat is given out Exothermic reaction. Work out the temperature change and decide if the reaction is exothermic or endothermic.
If it falls then the reaction is endothermic. Endothermic reactions involve the evolution of heat. If the temperature rises then the reaction is exothermic.
Endo means into so if a reaction is endothermic heat must be added during the reaction. If no H values are given the best way is to look at whats happening in the reaction. QUESTION 13 Whether a reaction is exothermic or endothermic is determined by.
Exothermic Reactions The opposite of an endothermic reaction is an exothermic reaction. An example is 6CO2 6H2O energy C6H12O6. A specialised piece of equipment called a calorimeter is used to accurately calculate enthalpy changes in a reaction using a similar method.
Whether a reaction is exothermic or endothermic is determined by whether a catalyst is present. Since breaking a bond requires energy this reaction must be endothermic. Identifying Exothermic Endothermic Reactions.
Neutralization burning a chemical fuel reactions deposition of dry ice respiration sulfuric acid solution in water and many more processes are examples. Regarding the question itself Im just nitpicking here but all reactions are reversible except for reactions where a product escapes from the system. Though chemical equations usually list only the matter components of a reaction you can also consider heat energy as a reactant or product.
A reaction that converts thermal energy to chemical energy heat is taken in Endothermic reaction. Examples of endothermic changes. Rinse out and dry the polystyrene cup.
The graph shows that the reactants have greater potential energy than the products. Discard the mixture in the sink with plenty of water. How do you determine experimentally of a reaction is endothermic or exothermic.
Look at the information given. If heat is released as a product of the reaction the reaction is exothermic. Chemical reactions transform both matter and energy.
Take up a quiz on Difference Between Endothermic and Exothermic Reactions. This means the difference in energy is. If the products side has a larger enthalpy the reaction is endothermic.
Decide whether various reactions are exothermic or endothermic by measuring temperature change in this practical. How reactant and product potential energies compare. So if the sum of the enthalpies of the reactants is greater than the products the reaction will be exothermic.
Examples of exothermic changes. If heat is listed on the side of. Formulae Sheet Stoichiometry rMmn Vmd or Vm Vnc c1V1 c2V2 Avogadros.
It releases energy to its surroundings in the form of light or heat. The reaction is exothermic. By calculating the enthalpy change in a chemical reaction you can determine whether the reaction is endothermic or exothermic.
The physical state of. Includes kit list and safety instructions. Monitor temperature change When energy is released in an exothermic reaction the temperature of the reaction mixture increases.
There are two methods for distinguishing between exothermic and endothermic reactions. In a chemical equation the location of the word heat can be used to quickly determine whether the reaction is endothermic or exothermic. The main difference between exothermic and endothermic reactions is that an endothermic reaction absorbs energy in the form of heat from its surroundings whereas an exothermic reaction releases energy to the surroundings.
When energy is absorbed in an endothermic reaction the temperature decreases. Im now looking at the course reader and this is pretty much what is in the explanation. The equation shows that 891 kJ of energy are released as a product.
Depiction of an energy diagram In a chemical reaction some bonds are broken and some bonds are formed. As such you can theoretically use le Chateliers principle to determine whether the reaction is exothermic or endothermic based on the change in equilibrium position when temperature changes. The overall enthalpy of the reaction is negative ie its an exothermic reaction where energy is released in the form of heat.
A thermometer could be used to track if a reaction produced heat exothermic or absorbed heat endothermic. If the enthalpy change listed for a reaction is negative then that reaction releases heat as it proceeds the reaction is exothermic exo- out. If it is difficult to get the reaction to proceed as is the case with many endothermic reactions and some exothermic reactions then we can perhaps study the reverse reaction which may well proceed spontaneously and easily.
If the enthalpy change listed for the reaction is positive then that reaction absorbs heat as it. A net release of energy from the reacting substances into the surroundings makes this an exothermic reaction.
Endothermic And Exothermic Reactions Enthalpy Youtube
How To Know Whether It S An Endothermic Or An Exothermic Reaction In Reversible Reactions Quora

Exothermic Reactions Release Energy Endothermic Reactions Consume Energy Exothermic Reaction Homeschool Science Chemistry
No comments for "Whether a Reaction Is Exothermic or Endothermic Is Determined by"
Post a Comment